Skyscraper length in record time
Our innovative serum stimulates hair follicles and natural processes to increase your eyelash and eyebrow growth cycle, resulting in longer, healthier, and thicker lashes.
Shop now90 days growth guarantee
Collection
Show all productsWhy Toplash?
Toplash is the best solution for promoting eyelash growth because for several reasons:
- Advanced formula: stimulates hair follicles, enhances pigmentation, and strengthens lashes
- Effective results: Toplash has a proven track record of delivering effective results
- Trusted brand: reputable brand with thousands of satisfied customers worldwide
Results in 90 days or your money back!
Why you should choose Toplash
Ingredients
Every Toplash product is certified safe and hypoallergenic.
European quality
Every Toplash product is manufactured in the European Union, in a COA and CPNP certified facility.
A natural alternative to lash extensions
Get Longer Lashes, For Less. There is no need for eyelash extensions.
90-day guarantee
If you are not completely satisfied with the results, we will refund the full purchase price.

The Evolution of Toplash
Toplash Cosmetics began its journey in Dublin, Ireland and has now expanded globally, with products available across the EU, US, South America, and Asia.
Our brand's mission is to enhance your natural beauty, and each of our products reflects this philosophy.
Our team of experts is dedicated to developing cosmetics that align with the principles of minimalism, sustainable consumption, and healthy formulas.
Read more aboutOur happy clients about Toplash

Follow us
Don't miss out on sales and special offers.
Toplash Eyelash and Eyebrow Growth Serum

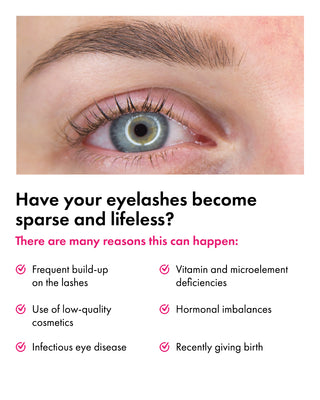
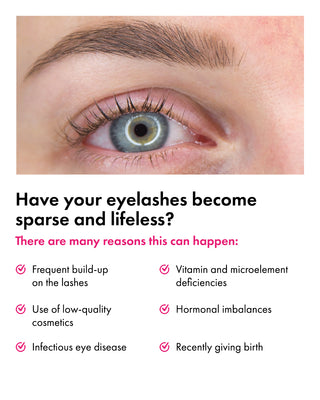
When it comes to enhancing the beauty of our eyes, luscious and long lashes can make a significant difference. However, not everyone is naturally blessed with voluminous eyelashes. This is where Toplash Eyelash and Eyebrow Growth Serum comes to the rescue. In this expert review, we will explore the benefits and results of using Toplash, the best natural lash growth serum available in the market today.
What is Toplash Eyelash and Eyebrow Growth Serum?
Toplash Eyelash and Eyebrow Growth Serum is a revolutionary product formulated to stimulate the growth of longer, thicker, and healthier lashes and eyebrows. This serum is created using a blend of natural ingredients that nourish and condition the hair follicles, promoting lash and brow growth.


The Benefits of Toplash Eyelash and Eyebrow Growth Serum
1. Enhanced Lash Length and Volume
Using Toplash consistently can lead to a remarkable improvement in lash length and volume. The powerful combination of ingredients in this serum stimulates the dormant hair follicles, encouraging them to grow and produce fuller lashes.
2. Healthier and Stronger Lashes
Toplash contains essential vitamins and proteins that provide nourishment to the lashes, making them stronger and more resilient. With regular use, you can say goodbye to brittle and weak lashes.
3. Visible Results in a Few Weeks
One of the most impressive aspects of Toplash is its effectiveness. Many users have reported noticeable results in as little as four weeks. However, individual results may vary, and it is recommended to continue using the serum for at least eight weeks to experience the full benefits.
4. Easy Application
Applying Toplash is a breeze. The serum comes with a thin brush applicator that allows for precise and effortless application along the lash line and eyebrows. Incorporating this into your daily beauty routine is quick and hassle-free.
5. Safe and Natural Formula
Toplash is made with natural ingredients and is free from harsh chemicals, making it safe for use on sensitive eyes and skin. The gentle formula minimizes the risk of irritation or allergies, ensuring a comfortable experience.
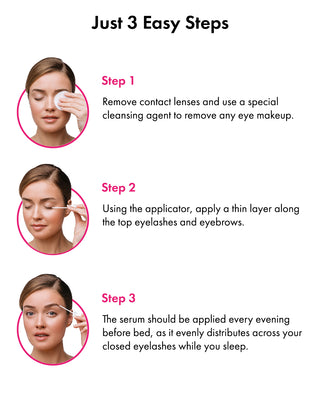
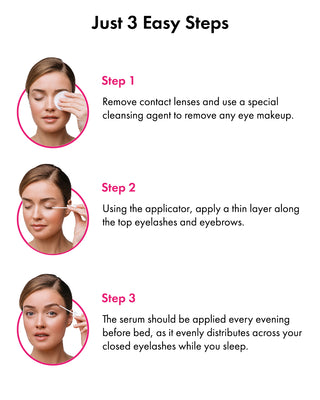
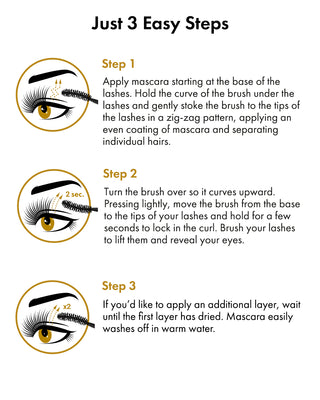
How to Use Toplash Eyelash and Eyebrow Growth Serum
Using Toplash is simple. Follow these steps for optimal results:
- Cleanse your face and remove any makeup.
- Ensure your lashes and eyebrows are dry.
- Apply a thin line of Toplash along the base of your upper lash line, similar to applying eyeliner.
- For eyebrows, apply a small amount of serum onto the sparse areas.
- Allow the serum to dry completely before applying any other products.
- For best results, use Toplash every evening before bed.
Toplash Eyelash and Eyebrow Growth Serum is undoubtedly the best natural lash growth serum available in the market. Its natural and safe formula, combined with its ability to deliver remarkable results, makes it a must-have beauty product for anyone desiring longer and thicker lashes. With consistent use, Toplash can transform your lashes, giving you the confidence and beauty you deserve.
So, if you've been dreaming of having stunning lashes, give Toplash a try. Experience the benefits of this incredible serum and say goodbye to mascaras and falsies. Get ready to embrace the beauty of your natural lashes!




What Results Can You Expect from Using Eyelash Growth Serum?
Using the best lash growth serum, such as Toplash Eyelash Growth Serum, can provide you with a range of benefits. Let's explore the positive aspects and the results you can expect from incorporating an eyelash growth serum into your beauty routine:
Longer and Thicker Lashes
One of the primary benefits of using an eyelash growth serum is the noticeable increase in lash length and thickness. The serum's active ingredients stimulate the hair follicles, encouraging them to produce longer and thicker lashes. With regular use, you can enjoy the appearance of fuller lashes.
Enhanced Lash Volume
The best and safest lash growth serum can also contribute to increased lash volume. As the serum nourishes and strengthens the lashes, they appear denser and more voluminous. This can create a striking and glamorous effect, making your eyes look more captivating.

Improved Lash Health
Using an Eyelash Growth Serum not only enhances the appearance of your lashes but also improves their overall health. The serum's nourishing ingredients provide vital nutrients to the lash follicles, promoting stronger and healthier lashes. You may notice a reduction in lash breakage and brittleness.
Reduced Lash Loss
If you have experienced lash shedding or thinning, an eyelash growth serum can help combat this issue. The serum helps to anchor the lashes, preventing premature shedding and promoting lash retention. This can result in a fuller lash line and minimize the appearance of sparse areas.
Convenience and Ease of Use
Using the best eyelash serum for longer lashes is a simple and convenient process. Typically, you apply the serum along the base of your upper lash line using a thin brush applicator. This can easily be incorporated into your daily beauty routine, requiring only a few seconds of your time.
Suitable for Various Conditions
Eyelash growth serums, including Toplash, can be used by individuals with different lash conditions or concerns. Some of the conditions and situations where eyelash growth serums can be beneficial include:
- Naturally short or sparse lashes
- Lash thinning due to aging
- Lash loss or damage from eyelash extensions or false lashes
- Lashes weakened by harsh mascaras or makeup removers
- Post-chemotherapy lash regrowth

Using an effective lash serum, such as Toplash, can provide you with longer, thicker, and healthier lashes. The serum's nourishing and stimulating ingredients work together to enhance lash length, volume, and overall lash health. By incorporating an eyelash growth serum into your routine, you can achieve the beautiful lashes you desire. Whether you have naturally short lashes, experience lash shedding, or want to revitalize your lash appearance, Toplash Eyelash Growth Serum can be your go-to solution.
Instructions for Effective Use of Toplash Eyelash and Eyebrow Growth Serum
Cleanse and Prep
Start by cleansing your face and ensuring that your lashes and eyebrows are free from makeup, oils, and dirt. Use a gentle cleanser or makeup remover specifically designed for the eye area. This step prepares the lashes and eyebrows for optimal absorption of the serum.
Application Technique
When applying Toplash Eyelash and Eyebrow Growth Serum, follow these steps:
- Ensure your lashes and eyebrows are completely dry before application.
- Dip the thin brush applicator into the serum, ensuring it is coated evenly.
- Starting from the inner corner of your upper lash line, apply a thin line of serum along the base of your lashes.
- For eyebrows, apply a small amount of serum onto the sparse areas or over the entire brow.
- Allow the serum to dry completely before applying any other products or cosmetics.
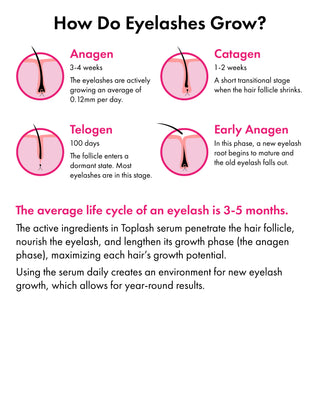
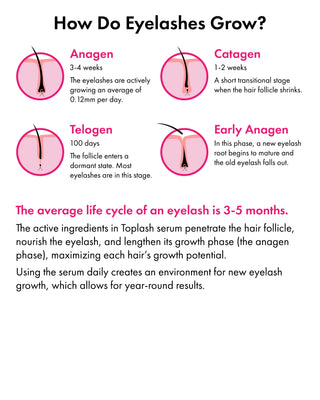
Duration of Results
Results from using Toplash Eyelash and Eyebrow Growth Serum may vary from person to person. Typically, visible improvements in lash length, volume, and thickness can be seen within 4-8 weeks of regular use. However, for optimal and long-lasting results, it is recommended to continue using the serum consistently for at least 12 weeks.



Discontinuation and Side Effects
If you choose to discontinue using eyelash serum for fuller lashes, the results achieved during the usage period are expected to gradually diminish. However, there are no known side effects associated with discontinuing the serum. It is important to note that Toplash is formulated with natural ingredients, minimizing the risk of adverse reactions.
Contraindications and Precautions
While Toplash is generally safe for use, it is advisable to take the following precautions:
- Avoid direct contact with the eyes. In case of accidental contact, rinse thoroughly with water.
- If you have a history of eye conditions or are currently experiencing eye infections or irritations, it is recommended to consult with a healthcare professional before using the serum.
- Pregnant or nursing individuals should consult with a healthcare professional before using the serum.
- Keep the serum out of reach of children.
Troubleshooting Tips
If you are not achieving the desired results with Toplash Eyelash and Eyebrow Growth Serum, consider the following steps:
- Ensure that you are using the serum consistently and following the recommended application technique.
- Check the expiration date of the product to ensure its effectiveness.
- Avoid using oil-based makeup or cleansers around the eye area, as they may interfere with the absorption of the serum.
- Examine your overall lifestyle and habits. Factors such as stress, diet, and certain medications can influence lash and brow growth.
- Consult with a cosmetologist or healthcare professional for personalized advice and guidance.
Compatibility with Other Products
Toplash Eyelash and Eyebrow Growth Serum is designed to work effectively on its own. However, if you wish to incorporate other eye cosmetics or beauty products into your routine, it is advisable to apply Toplash first and allow it to dry completely before applying other products. This ensures maximum absorption and effectiveness of the serum.




Transform Your Lashes: Before and After Lash Serum Using
Before: The Lash Struggle Was Real
Before stumbling upon this marvelous lash serum, I had tried countless mascara formulas and false lash applications, all in pursuit of achieving that coveted wide-eyed, fluttery lash look. Unfortunately, my natural lashes were far from impressive - short, sparse, and lacking the va-va-voom factor that I longed for. It seemed like I was doomed to live a life of mediocre lashes until I discovered the Best and safest lash growth serum - Toplash.
The Magical Transformation
The first thing that drew me to this lash serum was its promise of delivering noticeable results within a few weeks. Intrigued, I decided to give it a shot, and boy, am I glad I did! The application process was a breeze - just a thin line along the upper lash line before bed, and I was good to go. The serum itself had a lightweight, non-greasy texture, which was a big plus for me.

After a couple of weeks of consistent use, I started noticing subtle changes in my lashes. They appeared thicker and healthier, with fewer lash casualties during my makeup removal routine. By the fourth week, the results were undeniable. My lashes had grown significantly longer, and the sparse areas were filling in beautifully. It was as if I had finally discovered the secret to the lashes of my dreams.
The Confidence Boost
The transformative power of cheap lash growth serum extended beyond physical changes. With my newfound lashes, my confidence soared to new heights. I no longer felt the need to rely on heavy layers of mascara or struggle with tricky falsies. Even on minimal makeup days, my lashes commanded attention, framing my eyes and making them appear more vibrant and captivating. The compliments I received were a testament to the visible impact this lash serum had on my overall look.
So, if you're tired of battling with lackluster lashes and desire a natural solution that actually delivers results, the lash and brow building serum is an absolute game-changer. With its easy application and remarkable effectiveness, this serum has transformed my lashes from drab to fab, giving me the confidence to flaunt my fluttery fringe. Say goodbye to fake lashes and hello to your own beautiful, head-turning lashes. Trust me, your lash journey will never be the same again!


Toplash FAQ:
How to buy Toplash eyelash and eyebrow growth serum?
Now that you're aware of the incredible benefits offered by Toplash Serum, let's explore the hassle-free process of purchasing this remarkable product:
- Visit the Toplash Website: To begin your purchasing journey, head over to the official Toplash website at toplash.com.
- Explore the Product: Once on the website, navigate to the product section and locate the Toplash Eyelash and Eyebrow Growth Serum. Click on the product image or title to access detailed information about its benefits, ingredients, and usage instructions.
- Select Your Desired Quantity: Determine the quantity of Toplash Serum you wish to purchase. Toplash offers different package options to suit your needs, ensuring that you have an ample supply of this transformative serum.
- Add to Cart: After selecting your desired quantity, click on the "Add to Cart" button to proceed with your purchase.
- Review Your Order: On the cart page, carefully review your order to ensure the correct quantity of Toplash Serum has been added. You may also have the option to add any promotional codes or discounts at this stage.
- Proceed to Checkout: Once you're satisfied with your order, click on the "Proceed to Checkout" button to initiate the checkout process.
- Provide Shipping Information: Enter your shipping details, including your name, address, and contact information. Double-check the accuracy of the provided information to ensure a smooth delivery experience.
- Select Shipping Method: Choose your preferred shipping method from the available options. Toplash strives to provide efficient and reliable shipping services to deliver your order in a timely manner.
- Enter Payment Details: On the payment page, securely enter your payment information to complete the transaction. Toplash utilizes trusted payment gateways to safeguard your personal and financial details.
- Review and Place Order: Before finalizing your purchase, carefully review your order, shipping information, and payment details. Once you're confident that all information is accurate, click on the "Place Order" button to confirm your purchase.
Follow the provided instructions for application and make it a part of your daily beauty routine. Prepare to be amazed as you witness the transformative power of Toplash, and revel in the beauty of your enhanced eyelashes and eyebrows.
What delivery methods does Toplash have?
When you place an order with Toplash, we prioritize your satisfaction and aim to get your products delivered in the shortest possible time. For time-sensitive orders, we offer expedited delivery services that ensure your items reach you promptly. Our dedicated team works tirelessly to process and dispatch these orders swiftly, minimizing any delays.
For non-urgent orders, we provide a standard delivery option. While it may take slightly longer than expedited delivery, rest assured that we prioritize careful handling and secure packaging to safeguard your products during transit. Our shipping partners are renowned for their reliability, allowing us to maintain our commitment to timely delivery even with standard shipping.
Once you have successfully placed an order with Toplash, you will receive an order confirmation email containing the details of your purchase. Our fulfillment team immediately begins processing your order to ensure timely shipment. We double-check the accuracy of your order and verify the availability of each item to avoid any inconvenience.
To keep you informed about the progress of your order, we provide a convenient shipment tracking service. As soon as your order is dispatched, we share a tracking number with you, allowing you to monitor its journey from our warehouse to your doorstep. You can easily track your package online, enabling you to plan accordingly and stay updated on the estimated delivery date.
Write me the answer to the question "What are the methods of payment for Toplash products?" Please indicate the following payment methods: Visa, Mastercard, PayPal, American Express, Klarna, Apple Pay, Google Pay, Shop Pay.
Toplash offers a variety of payment methods to facilitate a convenient and secure shopping experience. The following payment options are available for purchasing Toplash products:
- Visa: Toplash accepts Visa cards, which are widely recognized and commonly used for online transactions. Customers can securely enter their Visa card details during checkout.
- Mastercard: Another popular payment option accepted by Toplash is Mastercard. Customers can use their Mastercard credit or debit cards to make purchases on the website.
- PayPal: Toplash also supports PayPal, a widely used online payment platform. Customers with a PayPal account can choose this option during checkout and securely complete their purchase using their PayPal balance or linked bank accounts or cards.
- American Express: Toplash accepts American Express cards as a form of payment. American Express cardholders can conveniently use their cards to make purchases on the Toplash website.
- Klarna: Toplash offers Klarna as a payment option, which allows customers to buy now and pay later. Klarna provides flexible payment plans, including installment payments, making it easier for customers to manage their purchases.
- Apple Pay: If you are using an Apple device, Toplash supports Apple Pay. This payment method allows customers to securely make purchases using their Apple devices, such as iPhones, iPads, or Macs, with their linked Apple Pay account or cards.
- Google Pay: Toplash also accepts Google Pay, a digital wallet platform developed by Google. Customers can use their Google Pay account or cards linked to it to complete their purchases securely.
- Shop Pay: Toplash provides the option to use Shop Pay, an accelerated checkout method for Shopify-based stores. With Shop Pay, customers can save their payment and shipping information, allowing for quicker and easier purchases.
Please note that availability of specific payment methods may vary depending on your location and the policies of Toplash. It is advisable to check the website's checkout page for the most up-to-date information on available payment options.
How often should I use Toplash Eyelash serum?
While there is no one-size-fits-all answer to how often you should use eyelash serum, a general guideline is to apply it once a day, preferably in the evening before bedtime. This allows the serum to work overnight, providing ample time for absorption and nourishment. However, always defer to the product instructions for the most accurate usage recommendations. The frequency of using eyelash serum depends on various factors, including the product instructions, serum concentration, individual sensitivity, desired results, and consistency. By considering these aspects and following the provided guidelines, you can maximize the benefits of your eyelash serum and achieve the beautiful, luscious lashes you desire. Remember to prioritize your comfort and consult a professional if you have any concerns or experience adverse reactions.
Are there any side effects when using Toplash Eyelash Serum?
As with any cosmetic product, Toplash Eyelash Serum carries the risk of side effects, albeit rare. It's vital to be aware of these potential outcomes to make an informed decision before incorporating the serum into your beauty routine. Here are some possible side effects:
- Eye Irritation and Redness. Individuals with sensitive eyes may experience mild irritation or redness upon using Toplash Eyelash Serum. This reaction typically subsides within a short period, as the eyes adjust to the product. However, if the irritation persists or worsens, it is advisable to discontinue use and consult an ophthalmologist.
- Dryness and Brittle Lashes. In rare cases, prolonged use of Toplash Eyelash Serum can lead to dryness and increased fragility of the lashes. This effect is more likely to occur when the product is not used as directed or is overapplied. To mitigate this, it is crucial to follow the recommended usage guidelines and avoid excessive application of the serum.
- Discoloration of the Iris. Though extremely rare, some individuals have reported changes in iris color after using Toplash Eyelash Serum. These changes are typically irreversible and occur due to the active ingredients in the serum affecting the melanin production in the eye. If you notice any alterations in iris color, it is vital to seek medical advice promptly.
- Allergic Reactions. While Toplash Eyelash Serum is generally well-tolerated, some individuals may develop an allergic reaction to the product. Allergies can manifest in various ways, including itching, swelling, and redness of the eyelids. If you experience any signs of an allergic reaction, it is crucial to cease using the serum immediately and consult a healthcare professional for appropriate treatment options.
While Toplash Eyelash Serum offers the allure of longer, more voluminous lashes, it's essential to be aware of the potential side effects and know how to handle any allergic reactions that may arise. By understanding the risks and taking necessary precautions, you can make an informed decision about incorporating this product into your beauty regimen. Remember, your eye health should always take precedence, so consult a professional if you experience any concerning symptoms.
The opinion of a cosmetologist

Branda M. Heim
Cosmetologist
Today, I want to share my professional opinion on Toplash Eyelash and Eyebrow Growth Serum. As a cosmetologist with years of experience, I've had the pleasure of trying out various products, and this serum has truly impressed me. Here's why I highly recommend it:
- Dual-action Formula: What sets Toplash Eyelash and Eyebrow Growth Serum apart is its ability to enhance both lashes and brows. The dual-action formula is designed to promote growth, improve density, and nourish these delicate areas. It's a fantastic all-in-one solution for achieving stunning lashes and perfectly shaped brows.
- Clinically Tested: Toplash takes its commitment to quality seriously. This serum has undergone rigorous clinical testing to ensure its safety and effectiveness. The results speak for themselves, with users experiencing visible improvements in lash length, thickness, and brow fullness.
- Natural Ingredients: We all want to prioritize the health of our lashes and brows, and that's where Toplash serum shines. It's formulated with natural ingredients that nourish and strengthen the hair follicles, supporting healthy growth. Say goodbye to harsh chemicals and hello to a more gentle approach to enhancing your natural beauty.
- Easy Application: Applying the serum is a breeze. The precise applicator allows for targeted application along the lash line and brow area, ensuring maximum absorption of the active ingredients. It seamlessly fits into your daily beauty routine, taking just a few seconds to apply.
- Positive Feedback: The Toplash Eyelash and Eyebrow Growth Serum has garnered positive feedback from both professionals and customers alike. Beauty experts and influencers have praised its effectiveness, and many users have shared their success stories, showcasing incredible lash and brow transformations. Finally, Toplash Eyelash and Eyebrow Growth Serum is a fantastic choice for anyone looking to enhance their lashes and brows. With its dual-action formula, clinically tested ingredients, and positive feedback, it's a reliable product that delivers impressive results. Don't wait any longer—give it a try and unlock the potential of your lashes and brows with Toplash!
Beauty blogger recommendation:
Elisabeth Buss
Cosmetics Blog
Hey beauties! I've got some exciting news for you all! I recently tried out this amazing Eyelash Growth Serum, and let me tell you, it's a total game-changer! If you've ever dreamt of having long, fluttery lashes that could rival even the most popular beauty influencers, then this serum is your secret weapon.
First things first, consistency is key! To see the best results, make sure you apply the serum daily. Trust me, your dedication will be rewarded with lashes that are beyond fabulous.
Here's a little pro tip: Make it part of your nighttime routine. Just imagine, as you're cozied up in bed, dreaming of all the amazing things you're going to conquer tomorrow, your lashes are getting nourished and ready to slay the day ahead. It's like a beauty sleep for your lashes!
When applying the serum, don't be shy. Take a small amount on the applicator and gently swipe it along your lash line. Think of it as a mini pampering session for your lashes. Oh, and don't forget your eyebrows! This serum works wonders on those too. You'll have brows that are #onfleek in no time!
Now, let's talk patience. I know we live in an era of instant gratification, but good things come to those who wait. It may take a few weeks to start noticing a significant difference, but believe me, it'll be worth it. Be patient, keep up with the routine, and get ready for the lash and brow transformation of a lifetime!
And finally, remember to flaunt those gorgeous lashes and brows! Once you start seeing the results, don't shy away from showing off your beautiful flutterers. Get ready for compliments and questions like, "What's your secret?" You can confidently share the magic of this Eyelash Growth Serum and spread the love!
So, my lovelies, if you're ready to unleash the power of stunning lashes and brows, go ahead and give this Eyelash Growth Serum a try. Trust me, you won't be disappointed. Prepare for a world of fluttery goodness and get ready to bat those lashes like never before. Happy lash and brow growing!
Customer Testimonials:
- Choosing a selection results in a full page refresh.
- Opens in a new window.




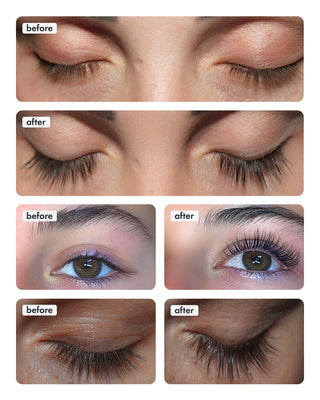






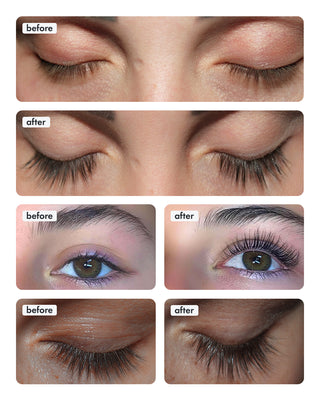











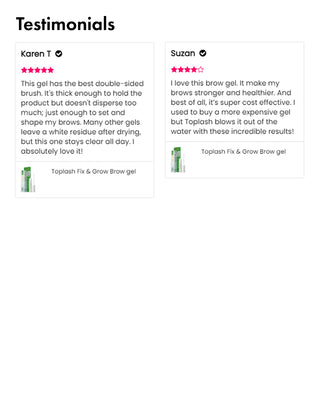






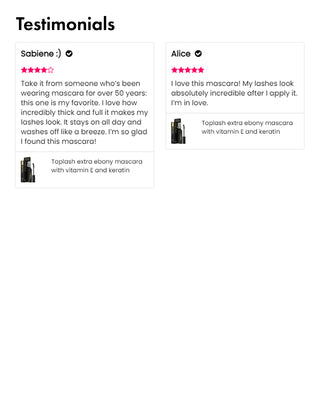






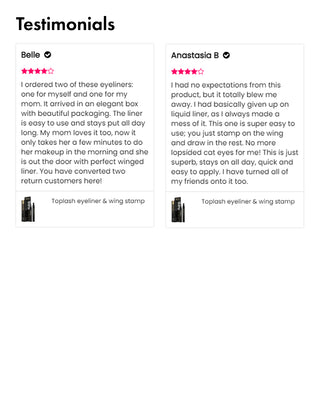
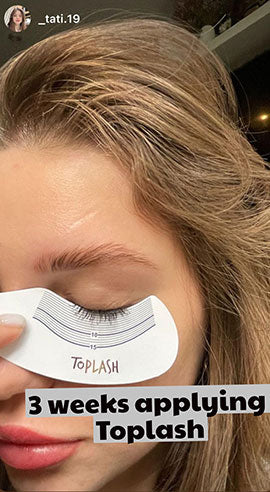

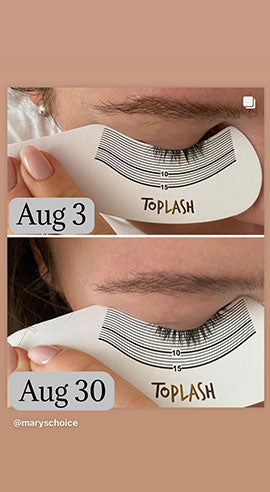
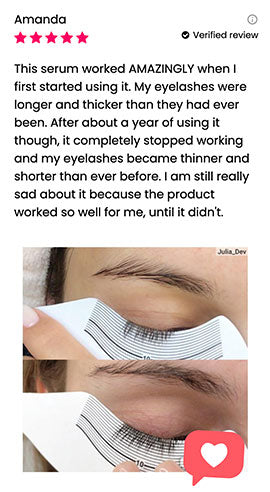
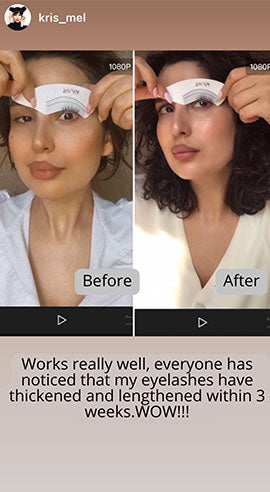


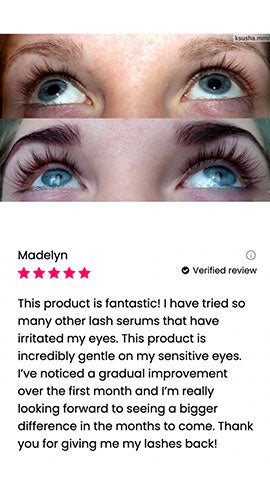


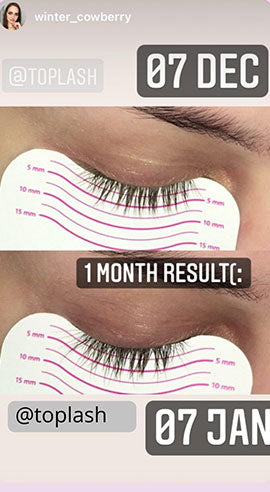

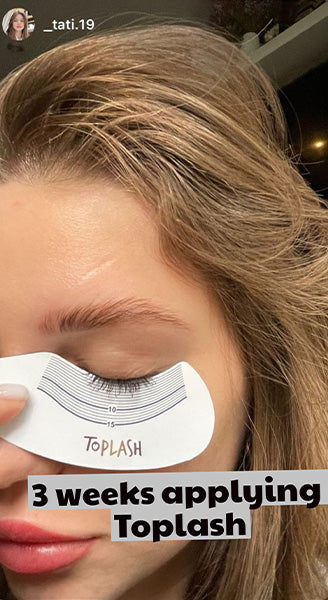
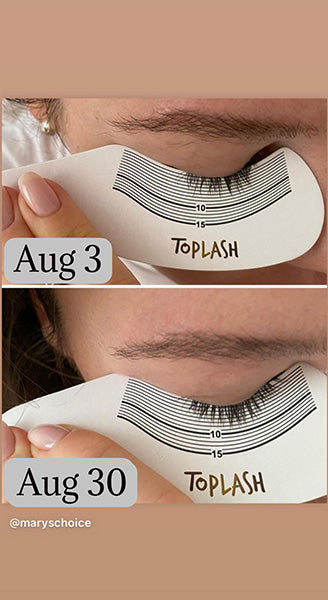
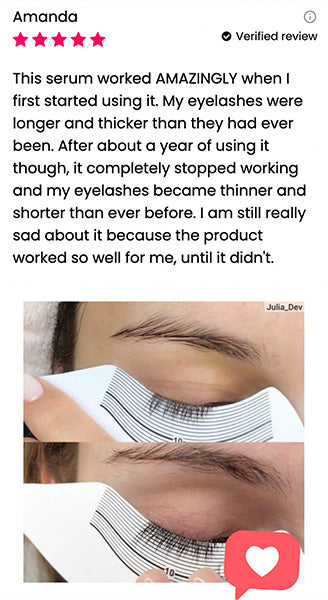
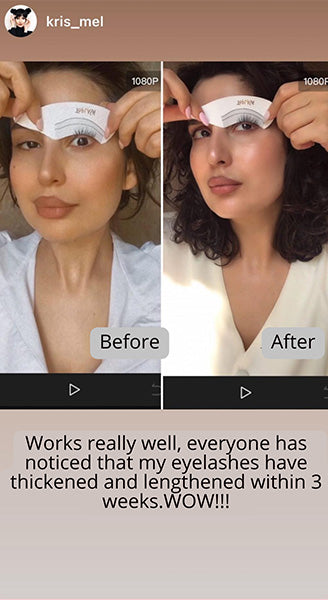


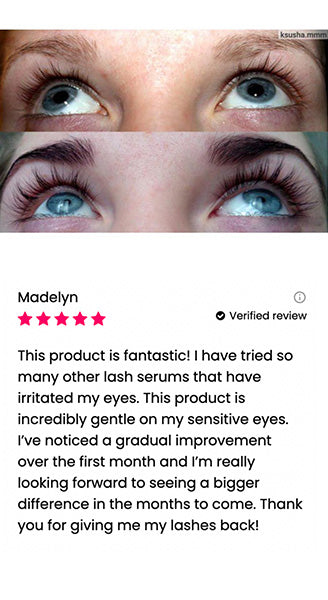


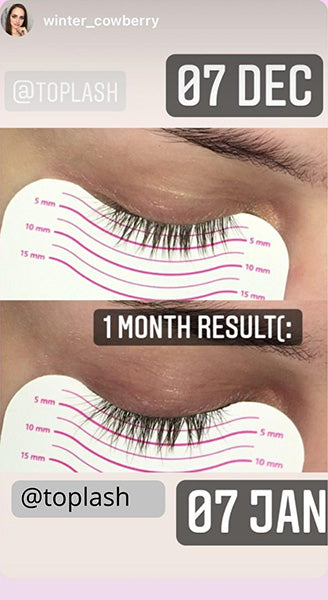

Wow, just wow! I have never been so amazed by a beauty product before. Toplash Eyelash Growth Serum is truly magical. I've been using it for just three weeks, and my lashes have transformed from barely there to long and luscious. It's like I'm wearing falsies every day, but they're all mine! I can't stop staring at my reflection in the mirror. Thank you, Toplash, for giving me the lashes of my dreams!
Emily Thompson, 32 y.o., Los Angeles
Toplash Eyelash and Eyebrow Booster is an absolute game-changer! I've always struggled with thin and sparse brows and lashes, but this product has completely transformed them. My lashes are now fluttery and full, while my brows have become beautifully defined. I can't help but admire my reflection every time I look in the mirror. Thank you, Toplash, for giving me the confidence to rock my natural beauty!
Isabella Martinez, 27 y.o., Houston